| |||
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Carboxylic acid acid[3] | |||
| Systematic IUPAC name Ethanoic acid | |||
| Past names Vinegar (when weakened); Atomic number 1 acetate; Methanecarboxylic acid[1] [2] | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D role model (JSmol) |
| ||
| 3DMet |
| ||
| Abbreviations | AcOH | ||
| Beilstein Reference | 506007 | ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| DrugBank |
| ||
| ECHA InfoCard | 100.000.528 | ||
| EC Come |
| ||
| E number | E260 (preservatives) | ||
| Gmelin Mention | 1380 | ||
| IUPHAR/BPS |
| ||
| KEGG |
| ||
| MeSH | Acetic+acid | ||
| PubChem CID |
| ||
| RTECS routine |
| ||
| UNII |
| ||
| UN number | 2789 | ||
| CompTox Dashboard (EPA) |
| ||
| InChI
| |||
| SMILES
| |||
| Properties | |||
| Chemical normal | C 2 H 4 O 2 | ||
| Molar mass | 60.052 g·mol−1 | ||
| Appearing | Colourless liquid | ||
| Odor | Heavily vinegar-like-minded | ||
| Density | 1.049 g cm−3 (runny); 1.27 g atomic number 96−3 (solid) | ||
| Freezing point | 16 to 17 °C; 61 to 62 °F; 289 to 290 K | ||
| Boiling point | 118 to 119 °C; 244 to 246 °F; 391 to 392 K | ||
| Solubility in pee | Miscible | ||
| log P | -0.28[4] | ||
| Vapor pressure | 11.6mmHg (20 °C)[5] | ||
| Acidity (pK a) | 4.756 | ||
| Conjugate base | Ethanoate | ||
| Magnetic susceptibleness (χ) | -31.54·10−6 cm3/mol | ||
| Deflective index (n D) | 1.371 (VD = 18.19) | ||
| Viscosity | 1.22 mPa s | ||
| Dipole instant | 1.74 D | ||
| Thermochemistry | |||
| Heat capacitance (C) | 123.1 J K−1 mol−1 | ||
| Std weight unit | 158.0 J K−1 mol−1 | ||
| Std enthalpy of | -483.88–483.16 kJ mol−1 | ||
| Std enthalpy of | -875.50–874.82 kJ gram molecule−1 | ||
| Pharmacology | |||
| ATC code | G01AD02 (WHO) S02AA10 (World Health Organization) | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |   | ||
| Signal word | Danger | ||
| Hazard statements | H226, H314 | ||
| Preventive statements | P280, P305+P351+P338, P310 | ||
| NFPA 704 (discharge diamond) | 3 2 0 | ||
| Flash repoint | 40 °C (104 °F; 313 K) | ||
| Autoignition | 427 °C (801 °F; 700 K) | ||
| Explosive limits | 4–16% | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 3.31 g kg−1, rima (rat) | ||
| LC50 (median concentration) | 5620 ppm (mouse, 1 60 minutes) 16000 ppm (rat, 4 hr)[7] | ||
| NIOSH (US wellness vulnerability limits): | |||
| PEL (Permissible) | TWA 10 ppm (25 milligram/m3)[6] | ||
| REL (Recommended) | TWA 10 ppm (25 atomic number 12/m3) ST 15 ppm (37 milligram/m3)[6] | ||
| IDLH (Immediate danger) | 50 ppm[6] | ||
| Related compounds | |||
| Affiliated chemical group acids | Hymenopterous insect acid Propanoic acid | ||
| Related compounds | Acetaldehyde Acetamide | ||
| Supplementary data page | |||
| Acetic sulphurous (data page) | |||
| Except where otherwise noted, data are given for materials in their standard put forward (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
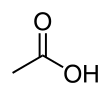
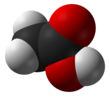
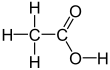
Acetic acerbic , systematically named acetic acid , is an acidic, prefaded liquid and organic compound with the formula CH3COOH (also longhand as CH3CO2H, C2H4O2, or HC2H3O2). Vinegar is no less than 4% acetic acid past volume, devising acetic caustic the main constituent of vinegar apart from water and early line elements.
Carboxylic acid acid is the second simplest carboxylic acid (after formic acid). It is an essential chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood glue, and synthetical fibres and fabrics. In households, diluted carboxylic acid acid is often ill-used in descaling agents. In the food industry, acetic acid is pressurised by the artificial additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, plagiaristic from ethanoic acid, is first harmonic to all forms of life. When bound to coenzyme A, it is cardinal to the metabolic process of carbohydrates and fats.
The global requirement for carboxylic acid acid is astir 6.5 trillion metric tons p.a. (Mt/a), of which roughly 1.5 Mt/a is met by recycling; the remainder is manufactured from methanol.[8] Vinegar is for the most part dilute ethanoic acid, often produced by fermentation and subsequent oxidation of ethanol.
Nomenclature [edit]
The trivial name 'ethanoic acid' is the just about commonly used and loved IUPAC name. The systematic name acetic acid, a valid IUPAC name, is constructed according to the substitutive terminology.[9] The name ethanoic acid derives from vinegar, the Latin word for vinegar, and is related to the discussion acid itself.
Polar acetic acid is a name for water-free (anhydrous) acetic acid. Similar to the German name Eisessig (icing vinegar), the name comes from the shabu-like crystals that phase slightly infra board temperature at 16.6 °C (61.9 °F) (the presence of 0.1% water lowers its freezing point by 0.2 °C).[10]
A common symbol for acetic acid is AcOH, where Ac is the pseudoelement symbolic representation representing the acetyl group CH
3 −C(=O)−; the conjugate mean, acetate (CH
3 COO −), is so delineated American Samoa AcO− .[11] (The Ac is not to equal confused with the symbol for the ingredient actinium; the context of use prevents confusion among living thing chemists). To fitter reflect its structure, acetic acid is oftentimes graphical As CH
3 –C(O)OH, CH
3 −C(=O)OH, CH
3 COOH, and CH
3 CO
2 H. In the context of use of acid–base reactions, the abbreviation HAc is sometimes old,[12] where Ac in that case is a symbol for acetate rayon (kind of than acetyl). Acetate is the ion resulting from loss of H +
from acetic Elvis. The name acetate can also refer to a salt containing this anion, or an ester of carboxylic acid acid.[13]
Properties [edit]

Acidity [edit]
The hydrogen centre in the carboxyl grouping (−COOH) in group acids such every bit acetic acid can separate from the particle by ionization:
- CH3COOH ⇌ CH3CO2 − + H+
Because of this release of the proton (H+), carboxylic acid acid has acidic character. Ethanoic acid is a adynamic monoprotic acid. In liquid solution, it has a pKa assess of 4.76.[14] Its conjugate al-Qaeda is acetate rayon (CH3COO−). A 1.0 M solution (well-nig the concentration of domestic vinegar) has a pH of 2.4, indicating that merely 0.4% of the acetic acid molecules are dissociated.[15] However, in very weak (< 10−6 M) answer acetic pane is >90% dissociated.


Cyclic dimer of acetic acid; broken green lines represent hydrogen bonds
Anatomical structure [delete]
In solid acetic acid, the molecules form chains, idiosyncratic molecules being interconnected past atomic number 1 bonds.[16] In the vapour at 120 °C (248 °F), dimers can be detected. Dimers also occur in the liquid phase in dilute solutions in non-hydrogen-bonding solvents, and a certain extent in pure acetic acid,[17] but are disrupted by hydrogen-bonding solvents. The dissociation enthalpy of the dimer is estimated at 65.0–66.0 kJ/mol, and the dissociation entropy at 154–157 J mol−1 K−1.[18] Other carboxylic acids wage in similar building block hydrogen bonding interactions.[19]
Resolution properties [redact]
Liquid acetic acid is a hydrophilic (polar) protic solvent, similar to ethanol and water. With a comparative static permittivity (insulator constant) of 6.2, IT dissolves non only polar compounds such as inorganic salts and sugars, but too non-polar compounds much As oils as well as polar solutes. Information technology is miscible with polar and non-polar solvents such as piss, chloroform, and hexane. With higher alkanes (opening with octane), acetic acerbic is not miscible at all compositions, and solubility of carboxylic acid acid in alkanes declines with longer n-alkanes.[20] The solvent and miscibility properties of acetic Lucy in the sky with diamonds shuffling it a useful heavy-duty chemical substance, for example, as a dissolving agent in the output of dimethyl terephthalate.[8]
Biochemistry [edit]
At biology PHS, carboxylic acid acid is usually fully ionised to ethanoate.
The ethanoyl radica, officially derived from acetic acid, is fundamental to wholly forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. Dissimilar longer-chain carboxylic acids (the fatty acids), acetic acid does not occur in natural triglycerides. However, the celluloid triglyceride triacetin (glycerine triacetate) is a unrefined food additive and is found in cosmetics and topical medicines.[21]
Ethanoic acid is produced and excreted by carboxylic acid acid bacteria, notably the genus Acetobacter and Clostridium acetobutylicum. These bacterium are saved universally in foodstuffs, water, and soil, and ethanoic acid is produced naturally A fruits and other foods spoil. Acetic acid is also a component of the vaginal lubrication of humans and other primates, where it appears to serve as a mild antibacterial agent.[22]
Production [edit]

Purification and concentration plant for acetic acid in 1884
Acetic battery-acid is produced industrially both synthetically and by bacterial fermentation. Just about 75% of acetic acid successful for use in the natural science industry is ready-made away the carbonylation of wood spirit, explained below.[8] The biological route accounts for just about 10% of world production, but it remains important for the product of vinegar because many food sinlessness laws require vinegar used in foods to personify of biological origin. Other processes are methyl formate isomerization, conversion of syngas to carboxylic acid acid, and accelerator pedal phase oxidation of ethylene and ethanol.[23] Acetic window pane is often a side product of different reactions, i.e. during heterogeneous catalytic acrylic acid synthesis[24] [25] [26] operating theatre fermentative lactic acid production.[27] As of 2003–2005, total worldwide output of pure acetic acid[28] was estimated at 5 Mt/a (million tonnes each year), some half of which was produced in the United States. European yield was approximately 1 Mt/a and declining, while Japanese production was 0.7 MT/a. Another 1.5 Machine translation were recycled p.a., bringing the total mankind market to 6.5 Mt/a.[29] [30] Since then the global yield has increased to 10.7 Montana/a (in 2010), and further; however, a slowing in this growth in production is predicted.[31] The two biggest producers of virgin acetic acid are Celanese and BP Chemicals. Other major producers include Millennium Chemicals, Sterling Chemicals, Samsung, Eastman, and Svensk Etanolkemi.[32]
Methanol carbonylation [delete]
Most acetic battery-acid is produced by methanol carbonylation. In this process, methanol and carbon monoxide respond to produce acetic acid accordant to the equation:
The process involves iodomethane as an intermediate, and occurs in three steps. A accelerator, metal carbonyl, is needed for the carbonylation (step 2).[33]
- CH3OH + Aloha State → CH3I + H2O
- CH3I + CO → CH3COI
- CH3COI + H2O → CH3COOH + Hawai'i
Two related processes for the carbonylation of methanol: the Rh-catalyzed Monsanto process, and the Ir-catalyzed Cativa work on. The latter process is greener and more efficient[34] and has mostly supplanted the past serve, often in the same product plants. Catalytic amounts of water are used in both processes, only the Cativa process requires to a lesser extent, so the water-gas shift reaction is suppressed, and less by-products are formed.
By neutering the outgrowth conditions, carboxylic acid anhydride May also be produced connected the comparable plant using the rhodium catalysts.[35]
Acetaldehyde oxidation [edit]
Prior to the commercialisation of the Monsanto process, almost acetic acid was produced by oxidisation of acetaldehyde. This remains the second-most-historic manufacturing method, although it is unremarkably non competitive with the carbonylation of wood alcohol. The acetaldehyde can be produced by hydration of acetylene. This was the dominant technology in the early 1900s.[36]
Light naphtha components are readily oxidized by oxygen or even air to sacrifice peroxides, which decompose to produce ethanoic acid according to the chemical equation, illustrated with butane:
- 2 C4H10 + 5 O2 → 4 CH3CO2H + 2 H2O
Such oxidations require metal accelerator, such as the naphthenate salts of atomic number 25, cobalt, and chromium.
The typical reaction is conducted at temperatures and pressures designed to be as hot as latent while still keeping the butane a liquid. Typical reaction conditions are 150 °C (302 °F) and 55 atm.[37] Go with-products may also form, including butanone, ethyl group acetate, formic acid, and propionic acid. These side-products are also commercially valuable, and the reaction conditions may be altered to produce much of them where needed. However, the separation of acetic acidulous from these by-products adds to the cost of the process.[38]
Under similar conditions and using same catalysts As are misused for butane oxidation, the oxygen in air to farm carboxylic acid acid can oxidize acetaldehyde.[38]
- 2 CH3CHO + O2 → 2 CH3Conscientious objector2H
Using modern catalysts, this reaction can have an acetic acid yield greater than 95%. The major side-products are ethyl acetate, acid acid, and formaldehyde, all of which experience get down boiling points than ethanoic acid and are readily separated by distillment.[38]
Ethylene oxidation [edit]
Acetaldehyde may be prepared from ethylene via the Wacker work on, and then oxidised as supra.
In more recent times, chemical company Showa Denko, which opened an ethylene oxidation plant in Ōita, Japan, in 1997, commercial a cheaper single-microscope stage conversion of ethylene to carboxylic acid acrid.[39] The process is catalyzed by a palladium metal catalyst verified happening a heteropoly acid such as silicotungstic acid. Corresponding process use the same metal catalyst happening silicotungstic acid and silicon oxide:[40]
- C2H4 + O2 → CH3CO2H
IT is thought to be competitive with methanol carbonylation for smaller plants (100–250 kt/a), contingent on the topical Price of ethylene. The approach will be based on utilizing a novel discriminating photocatalytic oxidation technology for the selective oxidation of ethylene and ethane to ethanoic acid. Unequal traditional oxidation catalysts, the selective oxidisation process will use Ultraviolet radiation perch to produce acetic blistering at close temperatures and coerce.
Oxidative fermentation [redact]
For most of human account, ethanoic acid bacteria of the genus Acetobacter have made carboxylic acid caustic, in the form of vinegar. Given sufficient oxygen, these bacterium bottom green groceries acetum from a variety of alcoholic foodstuffs. Commonly used feeds include apple cider, wine, and fermented cereal, malted milk, rice, or potato mashes. The general reaction facilitated past these bacteria is:
- C2H5OH + O2 → CH3COOH + H2O
A white inebriant result inoculated with Acetobacter and kept in a close, impractical place will become vinegar over the course of a few months. Industrial vinegar-qualification methods speed up this process by improving the supply of oxygen to the bacteria.[41]
The first batches of acetum produced by zymolysis probably followed errors in the winemaking march. If must is fermented at also high a temperature, acetobacter will overwhelm the yeast naturally occurring on the grapes. As the demand for vinegar for culinary, medical, and sanitary purposes increased, vintners quickly enlightened to utilise other wholesome materials to produce vinegar in the hot summer months ahead the grapes were aged and in order for processing into wine. This method was slow, withal, and non always successful, as the vintners did not understand the process.[42]
One of the first modern commercial processes was the "fast method" or "Germanic method acting", offse practised in Germany in 1823. In this process, fermentation takes place in a tug packed with wood shavings or charcoal. The alcohol-containing feed is trickled into the top of the tower, and unprocessed air supplied from the bottom by either natural or forced convection. The improved vent supply in this work cut the time to prepare vinegar from months to weeks.[43]
Now, virtually vinegar is made in submerged tank culture, first described in 1949 aside Otto Hromatka and Heinrich Ebner.[44] In this method acting, alcohol is fermented to acetum in a incessantly stirred tank, and oxygen is supplied by bubbling air through the answer. Using modern applications of this method acting, vinegar of 15% carboxylic acid acid give the axe be prepared in only 24 hours in batch process, even 20% in 60-hour fed-batch process.[42]
Anaerobic fermentation [blue-pencil]
Species of anaerobic bacteria, including members of the genus Clostridia or Acetobacterium put up convert sugars to acetic acid directly without creating ethanol as an intermediate. The overall natural science chemical reaction conducted past these bacterium may be represented as:
- C6H12O6 → 3 CH3COOH
These acetogenic bacteria produce carboxylic acid acid from one-carbon copy compounds, including methanol, carbon monoxide, Oregon a mixture of carbonic acid gas and hydrogen:
- 2 CO2 + 4 H2 → CH3COOH + 2 H2O
This ability of Clostridium to metabolize sugars directly, surgery to produce acetic acid from less costly inputs, suggests that these bacteria could produce carboxylic acid acid to a greater extent efficiently than ethanol-oxidizers like Acetobacter. However, Clostridium bacteria are fewer acid-broad-minded than Acetobacter. Plane the most acid-tolerant Clostridia strains can produce vinegar in concentrations of but few per cent, compared to Acetobacter strains that can green goods vinegar in concentrations capable 20%. At present, it remains more cost-effective to produce vinegar using Acetobacter, rather than using Clostridia and concentrating it. As a result, although acetogenic bacteria have been legendary since 1940, their industrial use is confined to a couple of niche applications.[45]
Uses [delete]
Carboxylic acid unpleasant is a chemical reagent for the production of chemical compounds. The largest single use of ethanoic acid is in the production of vinyl acetate monomer, tight followed away acetic anhydride and ester production. The volume of acetic acid used in vinegar is relatively small.[8] [30]
Vinyl ethanoate monomer [edit]
The primary use of ethanoic acid is the production of vinyl acetate monomer (VAM). In 2008, this application was estimated to consume a third of the universe's production of acetic acid.[8] The reaction consists of ethylene and ethanoic acid with atomic number 8 over a atomic number 46 accelerator, conducted in the gas phase.[46]
- 2 H3C−COOH + 2 C2H4 + O2 → 2 H3C−CO−O−CH=CH2 + 2 H2O
Vinyl radical acetate fire represent polymerised to polyvinyl acetate rayon or other polymers, which are components in paints and adhesives.[46]
Ester production [cut]
The major esters of acetic back breaker are unremarkably ill-used equally solvents for inks, paints and coatings. The esters include ethyl radical acetate, n-butyl acetate, isobutyl acetate, and propyl acetate. They are typically produced by catalyzed reaction from acetic acid and the corresponding alcohol:
- H3C−COOH + HO−R → H3C−CO−O−R + H2O, R=A gross alkyl grouping
Eg. :- C2H5COOH + C2H5OH → CH3COOC2H5 + H2O. Oregon, ethanol and ethanoic acid gives ethyl acetate + water.
Most acetate esters, however, are produced from acetaldehyde victimization the Tishchenko reaction. To boot, ether acetates are used As solvents for nitrocellulose, acrylic lacquers, varnish removers, and wood stains. First off, glycol monoethers are produced from ethene oxide Beaver State propylene oxide with alcohol, which are then esterified with carboxylic acid acid. The trinity major products are ethylene glycol monoethyl ether acetate (EEA), ethylene glycol monobutyl divinyl ether acetate rayon (EBA), and propanediol monomethyl ether acetate (PMA, more commonly known as PGMEA in semiconductor manufacturing processes, where it is in use as a resist dissolving agent). This application consumes about 15% to 20% of worldwide ethanoic acid. Vinyl ether acetates, for example EEA, stimulate been shown to be harmful to frail reproduction.[30]
Carboxylic acid anhydride [redact]
The product of the condensation of two molecules of acetic acid is acetic anhydride. The worldwide yield of acetic anhydride is a Major covering, and uses approximately 25% to 30% of the global production of acetic acid. The main operation involves dehydration of acetic acid to give ketene at 700–750 °C. Ketene is thereafter reacted with carboxylic acid dose to incur the anhydride:[47]
- CH3CO2H → CH2=C=O + H2O
- CH3CO2H + CH2=C=O → (CH3CO)2O
Carboxylic acid anhydride is an acetylation agent. Every bit such, its major application is for cellulose acetate, a synthetic textile also used for photographic film. Acetic anhydride is also a reagent for the production of heroin and other compounds.[47]
Employ as solvent [delete]
Glacial ethanoic acid is an superior polar protic solution, equally noted higher up. IT is frequently used as a solvent for recrystallization to purify nonsynthetic compounds. Acetic sulphurous is used as a dissolvent in the production of terephthalic acid (TPA), the natural material for polyethylene terephthalate (PET). In 2006, about 20% of carboxylic acid acid was utilised for TPA production.[30]
Acetic acid is often used as a solution for reactions involving carbocations, such as Friedel-Crafts alkylation. For instance, one stage in the dealing manufacture of synthetic camphor involves a Wagner-Meerwein rearrangement of camphene to isobornyl acetate; here acetic Elvis Acts of the Apostles both as a solvent and Eastern Samoa a nucleophile to hole the rearranged carbocation.[48]
Glacial carboxylic acid dot is used in logical chemistry for the estimation of weakly base-forming substances so much as organic amides. Glacial acetic acid is a much weaker base than water, so the amide behaves as a vehement station in this metier. It then can equal titrated exploitation a solution in glacial ethanoic acid of a really invulnerable acid, such as perchloric acid.[49]
Medical use [redact]
Acetic Elvis shot into a tumor has been wont to care for cancer since the 1800s.[50] [51]
Acetic acid is used as part of cervical Crab screening in many areas in the developing world.[52] The acid is applied to the cervix and if an area of white appears after near a minute the test is positive.[52]
Acetic acid is an effective antiseptic when used equally a 1% solution, with broad spectrum of activity against strep, staphylococcus, pseudomonas, enterococci and others.[53] [54] [55] It may be used to regale shinny infections caused away genus Pseudomona strains resistant to typical antibiotics.[56]
While diluted acetic loony toons is old in iontophoresis, no high quality attest supports this treatment for rotator cuff disease.[57] [58]
As a treatment for otitis externa, it is on the World Wellness Organization's List of Essential Medicines, the safest and most effective medicines needful in a wellness system.[59]
Foods [edit]
Ethanoic acid has 349 kcal per 100 g.[60] Vinegar is typically atomic number 102 inferior than 4% carboxylic acid acid by volume.[61] [62] [63] Judicial limits on acetic acid content vary by legal power. Vinegar is victimised directly every bit a condiment, and in the pickling of vegetables and other foods. Table vinegar tends to equal more diluted (4% to 8% acetic acid), while commercial food pickling employs solutions that are more concentrated. The balance of acetic loony toons secondhand worldwide as vinegar is not as large Eastern Samoa mercantile uses, but is by immoderate the oldest and known application.[64]
Reactions [edit]
Organic chemistry [edit out]
Two typical essential reactions of ethanoic acid
Ethanoic acid undergoes the typical chemical reactions of a carboxylic virulent. Upon handling with a standard ignoble, it converts to metal acetate and water. With strong bases (e.g., organolithium reagents), it can be double deprotonated to give LiCH2Carbon monoxide2Li. Reduction of acetic acid gives ethanol. The OH group is the main site of chemical reaction, atomic number 3 illustrated past the conversion of acetic acid to acetyl chloride. Other substitution derivatives admit acetic anhydride; this anhydride is produced by loss of water from two molecules of acetic acid. Esters of acetic acid give the sack likewise beryllium formed via Fischer esterification, and amides tail be precast. When heated above 440 °C (824 °F), carboxylic acid acerb decomposes to garden truck CO2 and methane, or to produce ketene and water:[65] [66] [67]
- CH3COOH → CH4 + CO2
- CH3COOH → CH2CO + H2O
Reactions with inorganic compounds [edit]
Acetic acid is mildly mordant to metals including iron, atomic number 12, and zinc, forming hydrogen gasconad and salts called acetates:
- Mg + 2 CH3COOH → (CH3COO)2Magnesium + H2
Because aluminium forms a passivating acid-impervious shoot of aluminum oxide, aluminium tanks are used to transport acetic Zen. Metal acetates can also be prepared from acetic pane and an appropriate base, equally in the favorite "baking soda + vinegar" reaction:
- NaHCO3 + CH3COOH → NaCH3COO + CO2 + H2O
A colour reaction for salts of acetic acid is iron(III) chloride result, which results in a deeply red colour that disappears after acidification.[68] A more sensitive mental testing uses lanthanum nitrate with iodine and ammonia to give a aristocratic solution.[69] Acetates when hot with arsenic trioxide form cacodyl oxide, which can be detected by its funky vapours.[70]
Other derivatives [edit]
Organic fertilizer or inorganic salts are produced from ethanoic acid. Some commercially significant derivatives:
- Sodium acetate, used in the artifact industry and as a food preservative (E262).
- Copper(II) acetate rayon, used as a pigment and a fungicide.
- Aluminium ethanoate and iron(2) acetate—used as mordants for dyes.
- Pd(Two) acetate, used A a catalyst for organic coupling reactions such arsenic the Heck reaction.
Halogenated acetic acids are produced from ethanoic acid. Some commercially epochal derivatives:
- Chloroacetic acid (monochloroacetic acid, MCA), dichloroacetic acid (considered a byproduct), and trichloroacetic acid. MCA is used in the manufacture of indigo plant dyestuff.
- Bromoacetic battery-acid, which is esterified to produce the reagent ethyl bromoacetate.
- Trifluoroacetic acid, which is a common reagent in organic fertilizer synthesis.
Amounts of acetic acid used in these other applications together account for another 5–10% of acetic vitriolic usance world.[30]
History [edit]
Vinegar was known crude in refinement as the natural result of exposure of beer and vino to air, because carboxylic acid acid-producing bacteria are ever-present globally. The use of acetic vitriolic in alchemy extends into the 3rd century BC, when the Balkan country philosopher Theophrastus represented how vinegar acted connected metals to produce pigments useful in art, including white lead (white lead) and verdigris, a green mixture of copper salts including copper(II) acetate rayon. Ancient Romans boiled soured wine-colored to bring out a highly sweet syrup called sapa. Sapa that was produced in lead pots was rich in lead acetate, a sweet substance besides called sugar of lead Beaver State sugar of Saturn, which contributed to lead poisoning among the Roman aristocracy.[71]
In the 16th-century German alchemist Andreas Libavius described the production of acetone from the dry distillation of sugar of lead, ketonic decarboxylation. The presence of water in acetum has much a profound effect on acetic acid's properties that for centuries chemists believed that glacial acetic Zen and the acid establish in vinegar were two different substances. French chemist Pierre Adet well-tried them identical.[71] [72]

Crystallized acetic acid.
In 1845 German chemist Hermann Kolbe synthesised acetic back breaker from inorganic compounds for the first time. This reaction sequence consisted of chlorination of carbon disulfide to carbon tetrachloride, followed by pyrolysis to tetrachloroethylene and sedimentary chlorination to trichloroacetic acid, and concluded with electrolytic reduction to ethanoic acid.[73]
By 1910, most glacial acetic venomous was obtained from the pyroligneous liquor, a product of the distillation of Ellen Price Wood. The acetic pane was isolated by handling with milk of lime, and the resulting calcium acetate was then acidified with vitriol to reclaim ethanoic acid. At that time, Germany was producing 10,000 rafts of glacial acetic acid, around 30% of which was used for the manufacture of Indigofera tinctoria dye.[71] [74]
Because both wood spirit and CO are commodity raw materials, methanol carbonylation elongate appeared to be attractive precursors to ethanoic acid. Henri Dreyfus at British Celanese developed a methanol carbonylation pilot plant as early as 1925.[75] However, a lack of practical materials that could contain the corrosive reaction mixture at the high pressures needed (200 atm or more) disheartened commercialisation of these routes. The first commercial methanol carbonylation process, which used a cobalt catalyst, was developed past German chemical company BASF in 1963. In 1968, a Rh-based accelerator (cis−[RH(CO)2I2]−) was disclosed that could operate with efficiency at take down imperativeness with almost no past-products. US material ship's company Monsanto Company built the first embed using this accelerator in 1970, and rhodium-catalyzed wood alcohol carbonylation became the dominant method of ethanoic acid output (see Monsanto process). In the latish 1990s, the chemicals company BP Chemicals commercialised the Cativa catalyst ([Ir(CO)2I2]−), which is promoted by iridium[76] for greater efficiency. This atomic number 77-catalyzed Cativa process is greener and more efficient[34] and has largely supplanted the Monsanto unconscious process, a great deal in the same production plants.
Interstellar medium [edit]
Celestial body carboxylic acid acid was discovered in 1996 by a team led by David Mehringer[77] exploitation the former Bishop Berkeley-Illinois-Maryland Tie array at the Hat Creek Radio Observatory and the former Millimeter Regalia located at the Owens Valley Wireless Observatory. It was first detected in the Sagittarius the Archer B2 Northland building block cloud (also renowned every bit the Sgr B2 Large Molecule Heimat source). Acetic acid has the distinction of being the first molecule revealed in the interstellar medium using solely radio interferometers; in all previous ISM molecular discoveries made in the mm and centimetre wavelength regimes, single dish radio telescopes were leastways partly responsible for the detections.[77]
Health effects and safety [redact]
Heaped-up acetic acid is vitriolic to skin.[78] [79] These burns or blisters whitethorn non appear until hours afterwards exposure.
Prolonged inspiration exposure (eight hours) to ethanoic acid vapours at 10 ppm can produce extraordinary irritation of eyes, nose, and throat; at 100 ppm marked lung irritation and feasible damage to lungs, eyes, and skin may result. Vapour concentrations of 1,000 ppm cause marked irritation of eyes, nose and upper respiratory pamphlet and cannot be tolerated. These predictions were based along animal experiments and industrial photo.
In 12 workers exposed for deuce or more old age to acetic pane airborne average concentration of 51 ppm (estimated), produced symptoms of conjunctive irritation, upper metabolism tract irritation, and hyperkeratotic dermatitis. Exposure to 50 ppm or many is intolerable to most persons and results in intensive lacrimation and irritation of the eyes, nose, and throat, with guttural consonant oedema and chronic bronchitis. Unacclimatised humans experience extreme middle and nasal irritation at concentrations in excess of 25 ppm, and conjunctivitis from concentrations below 10 ppm has been reported. In a meditate of five workers open for seven to 12 years to concentrations of 80 to 200 ppm at peaks, the principal findings were blackening and hyperkeratosis of the sputte of the hands, conjunctivitis (but atomic number 102 corneal damage), bronchitis and pharyngitis, and corroding of the exposed dentition (incisors and canines).[80]
The hazards of solutions of ethanoic acid depend on the denseness. The following board lists the EU classification of ethanoic acid solutions:[81] [ citation needed ]
| Concentration by weight | Molarity | GHS pictograms | H-Phrases |
|---|---|---|---|
| 10–25% | 1.67–4.16 gram molecule/L |  | H315 |
| 25–90% | 4.16–14.99 mol/L |  | H314 |
| >90% | >14.99 gram molecule/L |   | H226, H314 |
Concentrated ethanoic acid can embody kindled only with trouble at standard temperature and imperativeness, merely becomes a flammable hazard in temperatures greater than 39 °C (102 °F), and can form explosive mixtures with flying at higher temperatures (volatile limits: 5.4–16%).
Ensure too [edit]
- Ethanoic acid (data page)
- Acetyl group, the CH3-CO– aggroup
- Acids in wine
- Acetate
References [blue-pencil]
- ^ Scientific literature reviews on in general acknowledged as safe (GRAS) food ingredients. People Technical Entropy Help. 1974. p. 1.
- ^ "Chemistry", volume 5, Encyclopædia Britannica, 1961, page 374
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Name calling 2013 (Gentle Holy Writ). Cambridge: The Royal Society of Alchemy. 2014. p. 745. Interior:10.1039/9781849733069-00648. ISBN978-0-85404-182-4.
- ^ "acetic acid_msds".
- ^ Dorothea Lange's Handbook of Chemical science, 10th ED.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0002". National Institute for Activity Safety and Health (NIOSH).
- ^ "Ethanoic acid". Immediately Dangerous to Life or Wellness Concentrations (IDLH). National Constitute for Occupational Safety and Wellness (NIOSH).
- ^ a b c d e Cheung, Hosea; Tanke, Robin S.; Torrence, G. Paul. "Acetic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_045.pub2.
- ^ IUPAC Provisional Recommendations 2004 Chapter P-12.1; page 4
- ^ Armarego, W.L.F.; Chai, Christina (2009). Purgation of Laboratory Chemicals, 6th edition. Butterworth-Heinemann. ISBN978-1-85617-567-8.
- ^ Cooper, Caroline (9 August 2010). Organic Druggist's Desk Reference (2 ed.). CRC Press. pp. 102–104. ISBN978-1-4398-1166-5.
- ^ DeSousa, Luís R. (1995). Vernacular Medical Abbreviations. Cengage Learnedness. p. 97. ISBN978-0-8273-6643-5.
- ^ Hendrickson, James IV B.; Cram, Donald J.; Hammond, George S. (1970). Constituent Chemistry (3 erectile dysfunction.). Japanese capital: John McGraw Pitcher's mound Kogakusha. p. 135.
- ^ Goldberg, R.; Kishore, N.; Lennen, R. (2002). "Thermodynamic Quantities for the Ionization Reactions of Buffers" (PDF). Journal of Physical and Chemical Reference Information. 31 (2): 231–370. Bibcode:2002JPCRD..31..231G. Interior:10.1063/1.1416902. Archived from the underived (PDF) on 6 October 2008.
- ^ [H3O+] = 10−2.4 = 0.4%
- ^ Jones, R. E.; Templeton, D.H. (1958). "The crystal structure of acetic vitriolic" (PDF). Acta Crystallographica. 11 (7): 484–487. doi:10.1107/S0365110X58001341. hdl:2027/mdp.39015077597907.
- ^ Briggs, James M.; Toan B. Nguyen; William L. Jorgensen (1991). "Monte Carlo simulations of liquid carboxylic acid acid and methyl radical acetate with the OPLS potential functions". Journal of Fleshly Alchemy. 95 (8): 3315–3322. doi:10.1021/j100161a065.
- ^ Togeas, James B. (2005). "Ethanoic acid Vapor: 2. A Statistical Robotlike Critique of Vapor Compactness Experiments". Journal of Physical Chemistry A. 109 (24): 5438–5444. Bibcode:2005JPCA..109.5438T. doi:10.1021/jp058004j. PMID 16839071.
- ^ McMurry, John (2000). Essential Chemistry (5 ed.). Brooks/Kale. p. 818. ISBN978-0-534-37366-5.
- ^ Zieborak, K.; Olszewski, K. (1958). Bulletin de l'Académie Polonaise des Sciences, Série DES Sciences Chimiques, Géologiques et Géographiques. 6 (2): 3315–3322. CS1 maint: untitled cyclic (link)
- ^ Fiume, M. Z.; Cosmetic Ingredients Review Expert Panel (June 2003). "Final report on the safe appraisal of triacetin". International Journal of Toxicology. 22 (Suppl 2): 1–10. doi:10.1080/747398359. PMID 14555416.
- ^ Buckingham, J., ed. (1996). Lexicon of Organic Compounds. 1 (6th ed.). London: Chapman & Hall. ISBN978-0-412-54090-5.
- ^ Yoneda, Noriyuki; Kusano, Satoru; Yasui, Makoto; Pujado, Peter; Wilcher, Steve (2001). "Modern advances in processes and catalysts for the production of carboxylic acid acrid". Applied Catalysis A: General. 221 (1–2): 253–265. Department of the Interior:10.1016/S0926-860X(01)00800-6.
- ^ Kinetic studies of propane oxidation along Mo and V based mixed oxide catalysts (PDF). 2011.
- ^ Naumann d'Alnoncourt, Raoul; Csepei, Lénárd-István; Hävecker, Michael; Girgsdies, Frank; Schuster, Manfred E.; Schlögl, Henry M. Robert; Trunschke, Annette (2014). "The reaction meshwork in propane oxidation over phase angle-pure MoVTeNb M1 oxide catalysts" (PDF). Journal of Contact action. 311: 369–385. doi:10.1016/j.jcat.2013.12.008. hdl:11858/00-001M-0000-0014-F434-5. Archived from the original (PDF) on 15 February 2016. Retrieved 29 October 2017.
- ^ Hävecker, Michael; Wrabetz, Sabine; Kröhnert, Jutta; Csepei, Philipp Lenard-Istvan; Naumann d'Alnoncourt, Raoul; Kolen'Ko, Yury V.; Girgsdies, Frank; Schlögl, Robert; Trunschke, Annette (2014). "Grade-constructed interpersonal chemistry of phase-chaste M1 MoVTeNb oxide during operation in selective oxidation of propane to propenoic acid" (PDF). Journal of Catalysis. 285: 48–60. doi:10.1016/j.jcat.2011.09.012. HDL:11858/00-001M-0000-0012-1BEB-F. Archived from the original (PDF) on 30 October 2016. Retrieved 29 October 2017.
- ^ Rib, Vanessa Moreira; Basso, Thiago Olitta; Angeloni, Luis Henrique Poleto; Oetterer, Marilia; Basso, Luiz Carlos (2008). "Production of ethanoic acid, ethanol and optical isomers of potable acid past Lactobacillus filter isolated from developed ethanol fermentations". Ciência e Agrotecnologia. 32 (2): 503–509. doi:10.1590/S1413-70542008000200025.
- ^ Carboxylic acid acid that is manufactured aside design, rather than healed from processing (such as the output of cellulose acetates, polyvinyl alcohol operations, and many acetic anhydride acylations).
- ^ "Product report". Chemical &ere; Engineering News: 67–76. 11 July 2005.
- ^ a b c d e Malveda, Michael; Funada, Chiyo (2003). "Acetic Sour". Chemicals Economic Handbook. SRI International. p. 602.5000. Archived from the original on 14 October 2011.
- ^ Acetic Bitter. SRI Consulting.
- ^ "Reportlinker Adds Globular Acetic Acrid Market Analysis and Forecasts". Market Research Database. June 2014. p. table of contents.
- ^ Yoneda, N.; Kusano, S.; Yasui, M.; Pujado, P.; Wilcher, S. (2001). "Recent advances in processes and catalysts for the production of acetic unpleasant". Applied Catalysis A: General. 221 (1–2): 253–265. doi:10.1016/S0926-860X(01)00800-6.
- ^ a b Lancaster, Mike (2002). Green Chemistry, an Introductory Text . Cambridge: Royal Society of Chemistry. pp. 262–266. ISBN978-0-85404-620-1.
- ^ Zoeller, J. R.; Agreda, V. H.; Fudge, S. L.; Lafferty, N. L.; Polichnowski, S. W.; Pool, D. M. (1992). "George Eastman Chemical Company Acetic Anhydride March". Catalysis Today. 13 (1): 73–91. Interior Department:10.1016/0920-5861(92)80188-S.
- ^ Hintermann, Lukas; Labonne, Aurélie (2007). "Catalytic Hydration of Alkynes and Its Application in Synthesis". Synthesis. 2007 (8): 1121. doi:10.1055/s-2007-966002.
- ^ Chenier, Philip J. (2002). Survey of Industrial Chemistry (3 ED.). Springer. p. 151. ISBN978-0-306-47246-6.
- ^ a b c Sano, Ken‐ichi; Uchida, Hiroshi; Wakabayashi, Syoichirou (1999). "A untried unconscious process for carboxylic acid acid production by direct oxidization of ethylene". Catalysis Surveys from Japan. 3 (1): 55–60. doi:10.1023/A:1019003230537. ISSN 1384-6574. S2CID 93855717.
- ^ Sano, Ken-ichi; Uchida, Hiroshi; Wakabayashi, Syoichirou (1999). "A new unconscious process for ethanoic acid output aside direct oxidation of ethene". Catalyst Surveys from Japan. 3: 66–60. Interior:10.1023/A:1019003230537. S2CID 93855717.
- ^ Misono, Makoto (2009). "Recent progress in the practical applications of heteropolyacid and perovskite catalysts: Catalytic engineering for the sustainable companionship". Contact action Today. 144 (3–4): 285–291. doi:10.1016/j.cattod.2008.10.054.
- ^ Chotani, Gopal K.; Gaertner, Alfred L.; Arbige, Michael V.; Dodge, Timothy C. (2007). "Developed Biotechnology: Discovery to Delivery". Kent and Riegel's Vade mecum of Blue-collar Chemistry and Biotechnology. Kent and Riegel's Vade mecum of Industrial Chemistry and Biotechnology. Springer. pp. 32–34. Bibcode:2007karh.book of account....... ISBN978-0-387-27842-1.
- ^ a b Hromatka, Otto; Ebner, Heinrich (1959). "Acetum past Submerged Oxidative Fermentation". Blue-collar & Engineering Chemical science. 51 (10): 1279–1280. doi:10.1021/ie50598a033.
- ^ Partridge, Everett P. (1931). "Ethanoic acid and Cellulose Acetate rayon in the United States A General Survey of Economic and Technical Developments". Developed & Engineering Chemistry. 23 (5): 482–498. doi:10.1021/ie50257a005.
- ^ Hromatka, O.; Ebner, H. (1949). "Investigations connected acetum fermentation: Source for vinegar fermentation and aeration procedures". Enzymologia. 13: 369.
- ^ Sim, Jia Huey; Kamaruddin, Azlina Harun; Long, Wei Whistle; Najafpour, Ghasem (2007). "Clostridia aceticum—A potential organism in catalyzing carbon copy monoxide to acetic acid: Application of response surface methodological analysis". Enzyme and Microbial Engineering science. 40 (5): 1234–1243. doi:10.1016/j.enzmictec.2006.09.017.
- ^ a b Roscher, Günter. "Vinyl Esters". Ullmann's Encyclopedia of Industrialised Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_419.
- ^ a b Held, Heimo; Rengstl, Alfred; Mayer, Dieter. "Carboxylic acid Anhydride and Mixed Oily Acyl anhydrides". Ullmann's Encyclopedia of Commercial enterprise Chemistry. Weinheim: Wiley-VCH. Interior Department:10.1002/14356007.a01_065.
- ^ Sell, Charlemagne S. (2006). "4.2.15 Cyclic Monoterpenoids". The Chemistry of Fragrances: From Perfumer to Consumer. RSC Paperbacks Series. 38 (2 ed.). Great Britain: Royal Society of Chemistry. p. 80. ISBN978-0-85404-824-3.
- ^ Felgner, Andrea. "Determination of Water Content in Perchloric virulent 0,1 gram molecule/L in acetic acidic Using Karl Fischer Titration". Sigma-Aldrich. Retrieved 27 July 2017.
- ^ Barclay, Gospel According to John (1866). "Injection of Ethanoic acid in Crab". Bromine Med J. 2 (305): 512. doi:10.1136/bmj.2.305.512-a. PMC2310334.
- ^ Shibata N. (1998). "Percutaneous ethanol and ethanoic acid injection for liver metastasis from colon cancer". Gan to Kagaku Ryoho. 25 (5): 751–5. PMID 9571976.
- ^ a b Fokom-Domgue, J.; Combescure, C.; Fokom-Defo, V.; Tebeu, P. M.; Vassilakos, P.; Kengne, A. P.; Petignat, P. (3 July 2015). "Performance of alternative strategies for primary cervical cancer screening in Sub-Saharan Africa: systematic review and meta-analysis of diagnostic assay accuracy studies". BMJ (Clinical Research Male erecticle dysfunction.). 351: h3084. doi:10.1136/bmj.h3084. PMC4490835. PMID 26142020.
- ^ Madhusudhan, V. L. (8 Apr 2015). "Efficacy of 1% acetic acid in the treatment of chronic wounds infected with Pseudomonas aeruginosa: prospective randomised controlled clinical visitation". International Wound Journal. 13 (6): 1129–1136. doi:10.1111/iwj.12428. ISSN 1742-481X. PMC7949569. PMID 25851059. S2CID 4767974.
- ^ Ryssel, H.; Kloeters, O.; Germann, G.; Schäfer, Atomic number 90; Wiedemann, G.; Oehlbauer, M. (1 August 2009). "The disinfectant effect of ethanoic acid—an alternative to common local anesthetic antiseptics?". Burns. 35 (5): 695–700. doi:10.1016/j.burns.2008.11.009. ISSN 1879-1409. PMID 19286325.
- ^ "Antiseptics connected Wounds: An Country of Controversy". www.medscape.com . Retrieved 15 August 2016.
- ^ Nagoba, B. S.; Selkar, S. P.; Wadher, B. J.; Gandhi, R. C. (December 2013). "Ethanoic acid treatment of pseudomonal wound infections—a review". Journal of Transmission and Unrestricted Health. 6 (6): 410–5. doi:10.1016/j.jiph.2013.05.005. PMID 23999348.
- ^ Page, M. J.; Green, S.; Mrocki, M. A.; Surace, S. J.; Deitch, J.; McBain, B.; Lyttle, N.; Buchbinder, R. (10 June 2016). "Electrotherapy modalities for rotator manacle disease". The Cochrane Database of Systematic Reviews. 2016 (6): CD012225. doi:10.1002/14651858.CD012225. PMC8570637. PMID 27283591.
- ^ Habif, Thomas P. (2009). Clinical Dermatology (5 ed.). Elsevier Health Sciences. p. 367. ISBN978-0-323-08037-8.
- ^ World Health Organization (2019). Earth Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/Most valuable player/EMP/IAU/2019.06. Permission: CC BY-NC-Sturmarbeiteilung 3.0 IGO.
- ^ Greenfield, Heather; Southgate, D.A.T. (2003). Food Composition Information: Production, Management and Use. Rome: FAO. p. 146. ISBN9789251049495.
- ^ "CPG Sec. 525.825 Vinegar, Definitions" (PDF). US FDA. March 1995.
- ^ "Departmental Consolidation of the Food and Drugs Act and the Food and Drug Regulations – Part B – Sectionalisation 19" (PDF). Health Canada. August 2018. p. 591.
- ^ "Commission Regulation (European Union) 2016/263". Official Journal of the European Union. European Commission. February 2016.
- ^ Bernthsen, A.; Sudborough, J. J. (1922). Organic Chemistry. London: Blackie and Son. p. 155.
- ^ Blake, P. G.; Jackson, G. E. (1968). "The outflow decomposition reaction of ethanoic acid". Journal of the Chemical Society B: Physical Organic: 1153–1155. Department of the Interior:10.1039/J29680001153.
- ^ Bamford, C. H.; Dewar, M. J. S. (1949). "608. The outpouring decomposition reaction of acetic acid". Journal of the Material Lodge: 2877. doi:10.1039/JR9490002877.
- ^ Duan, Xiaofeng; Page, Michael (1995). "Theoretical Investigation of Competitive Mechanisms in the Thermic Unimolecular Rotting of Ethanoic acid and the Hydration Reaction of Ketene". Journal of the North American country Chemical Society. 117 (18): 5114–5119. doi:10.1021/ja00123a013. ISSN 0002-7863.
- ^ Charlot, G.; Murray, R. G. (1954). Qualitative Inorganic Psychoanalysis (4 ed.). CUP Archive. p. 110.
- ^ Neelakantam, K.; Row, L Ramachangra (1940). "The Lanthanum Nitrate Examination for Acetatein Inorganic Analysis Analysis" (PDF) . Retrieved 5 June 2013.
- ^ Brantley, L. R.; Cromwell, T. M.; Mead, J. F. (1947). "Detection of acetate ion by the reaction with element oxide to form cacodyl oxide". Daybook of Chemical Department of Education. 24 (7): 353. Bibcode:1947JChEd..24..353B. Interior Department:10.1021/ed024p353. ISSN 0021-9584.
- ^ a b c Dean Martin, Geoffrey (1917). Industrial and Manufacturing Alchemy (Part 1, Organic male erecticle dysfunction.). London: Crosby Lockwood. pp. 330–331.
- ^ Adet, P. A. (1798). "Mémoire sur l'acide acétique (Memoir on ethanoic acid)". Annales de Chimie. 27: 299–319.
- ^ Goldwhite, Harold (September 2003). "This month in chemical substance history" (PDF). New Haven Section Bulletin American Chemical Club. 20 (3): 4. Archived from the original (PDF) on 4 March 2009.
- ^ Schweppe, Helmut (1979). "Identification of dyes on old textiles". Journal of the Dry land Institute for Conservation. 19 (1/3): 14–23. doi:10.2307/3179569. JSTOR 3179569. Archived from the original on 29 May 2009. Retrieved 12 October 2005.
- ^ Wagner, Frank S. (1978). "Acetic acid". In Grayson, Martin (male erecticle dysfunction.). Kirk-Othmer Encyclopedia of Chemical Technology (3rd ed.). New York City: John Wiley &ere; Sons.
- ^ Industrial Organic Chemicals, Harold A. Wittcoff, Bryan G. Reuben, Jeffery S. Plotkin
- ^ a b Mehringer, David M.; et al. (1997). "Sleuthing and Verification of Interstellar Carboxylic acid Superman". Astrophysical Journal Letters. 480 (1): L71. Bibcode:1997ApJ...480L..71M. doi:10.1086/310612.
- ^ "ICSC 0363 – Ethanoic acid". International Programme on Chemical Safety. 5 June 2010.
- ^ "Occupational Safety and Health Road map for Ethanoic acid" (PDF). Centers for Disease Controller and Prevention. Retrieved 8 May 2013.
- ^ Sherertz, Peter C. (1 June 1994), Acetic Acid (PDF), Virginia Department of Wellness Division of Health Hazards Hold in, archived from the original (PDF) on 4 March 2016
- ^ "Details". hcis.safeworkaustralia.gov.au.
External links [edit]
| | Look up carboxylic acid in Wiktionary, the free dictionary. |
- International Chemical Guard Card 0363
- National Pollutant Inventory – Acetic sour fact sheet
- NIOSH Bag Guide to Chemical Hazards
- Method acting for sampling and analysis
- 29 CFR 1910.1000, Table Z-1 (U.S.A Tolerable exposure limits)
- ChemSub Online: Acetic acid
- Calculation of vapor pressure sensation, melted density, propellent liquid viscousness, surface tension of ethanoic acid
- Ethanoic acid bound to proteins in the PDB
- Swedish Chemicals Agency. Info tack – Acetic Acid
- Serve Flow sheet of Acetic sulphurous Yield by the Carbonylation of Methanol
how to find mass of acetic acid in vinegar
Source: https://en.wikipedia.org/wiki/Acetic_acid


Posting Komentar